E-methanol – the future fuel?

The search for tomorrow’s fuel is under way in many places. Gothenburg company Liquid Wind is planning to build the world’s biggest production plant for e-methanol, which will be produced using wind power and carbon dioxide emissions from the pulp and paper industry.
IN CONTRAST TO petroleum-based fuels such as bunker oil and LNG, methanol comprises only one type of molecule: CH3OH. This means that methanol, when burned, just like its close relative ethanol, produces virtually no sulphur or particulates and only emits low levels of nitrogen oxide.
However, the methanol produced today is largely made from fossil sources – either natural gas or in some cases from coal, as in China. But if methanol could be produced from non-fossil sources, much would be gained. This is the business idea behind the Liquid Wind operation in Gothenburg.
Water soluble – and easy to handle
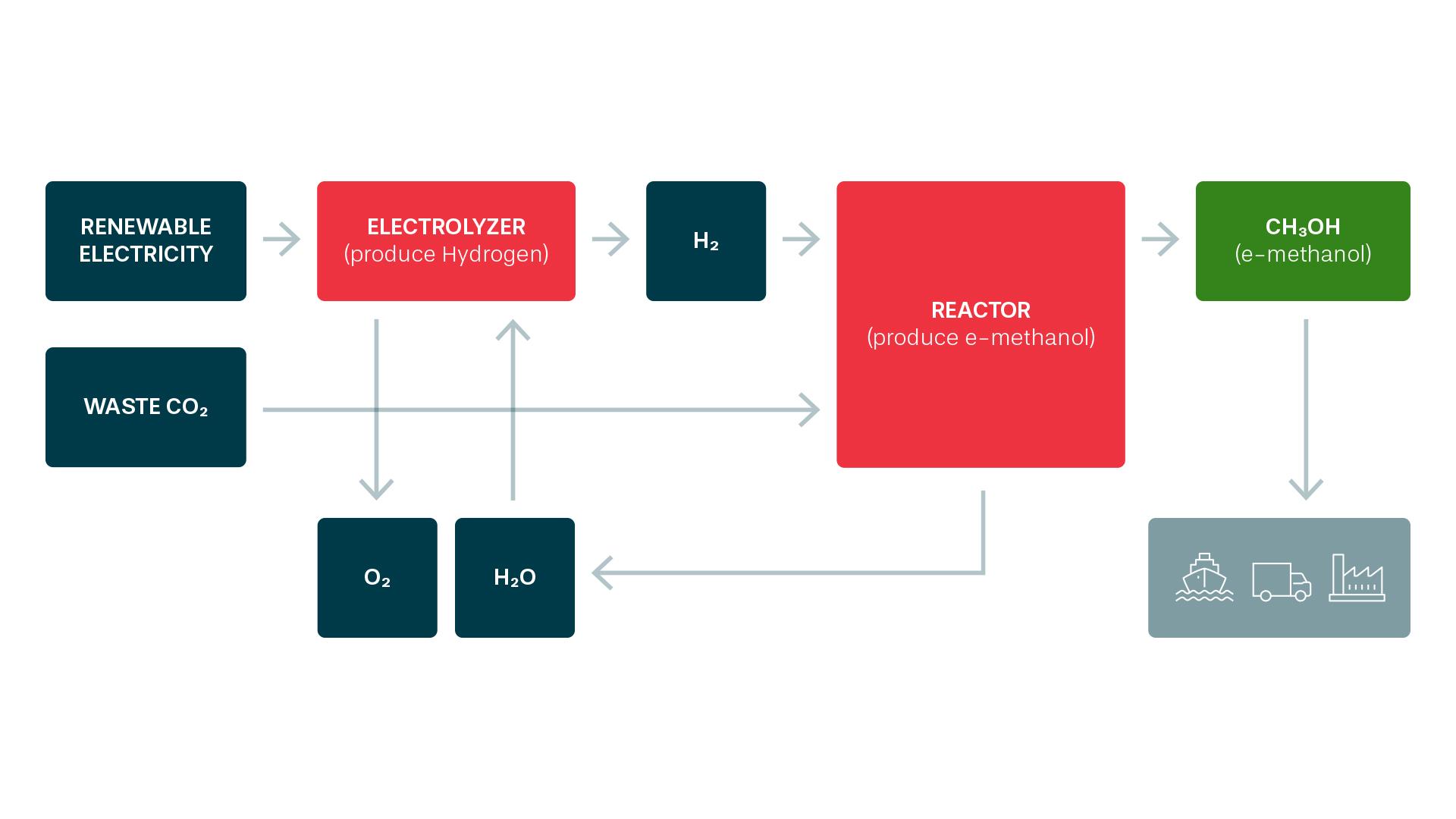
The basic principle is simple chemistry. Water is split into hydrogen and oxygen with the aid of electricity from wind or hydro power. The hydrogen is then combined with carbon dioxide from the paper industry to form methanol. To distinguish the end product from conventionally produced methanol, it’s called e-methanol, where e- stands for the electricity used in production.
“Methanol is an excellent fuel, especially for ships. It’s a liquid at room temperature, unlike LNG or pure hydrogen, which makes handling much easier. It’s water-soluble, which means that any leaks are quickly diluted and broken down in natural processes. Its use results in very low emissions of sulphur, particulates and NOx. Furthermore, e-methanol is produced from renewable raw materials using green energy,” says Thomas Stenhede, Senior Technical Advisor at Liquid Wind, and also one of its founders.

Production plant planned for Norrland
Liquid Wind plans to open an e-methanol plant for full-scale production in 2023. Thomas Stenhede is reluctant to say precisely where it will be located, as not all environmental permits are in place, but it will be somewhere along the Norrland coast next to a paper mill.
“The area has a plentiful supply of renewable wind electricity, carbon dioxide and water, and enjoys a well-developed infrastructure. The first plant will be 50 megawatts and have an annual production of 45,000 tonnes of e-methanol made using 70,000 tonnes of carbon dioxide from the paper mill. This will reduce the need for oil by over 20,000 tonnes per year,” says Thomas Stenhede.
New safety procedures needed
Methanol also has some disadvantages.
“It’s toxic, like any other fuel, and it will require different safety procedures. For example, methanol burns with an invisible flame. This is because it forms no particulates, which is fabulous in terms of the environment, but can be dangerous if you cannot locate the fire in an incident. So this will require training and new procedures,” says Thomas Stenhede.
Another disadvantage is the energy content, which is only about half that of today’s ship fuels.
“However, this is not a direct disadvantage on ships as they have plenty of space for fuel tanks and must anyway take on ballast. What’s more, the tanks can be made in any shape to fit the interior, unlike LNG tanks, which must be cylindrical.”
The initial price of e-methanol will be about twice that of today’s low cost fossil fuels. However, Liquid Wind believes they will achieve partiy with fossil fues by 2030. Thomas Stenhede believes methanol will become increasingly attractive as more stringent emission standards are imposed.
WALLENIUS SOL is following developments of all fossil-free fuels with great interest.
In Addition

Methanol – advantages and disadvantages
What are the advantages and disadvantages of methanol as a fuel? We spoke to Selma Brynolf, scientist at the Department of Mechanics and Maritime Sciences at Chalmers University of Technology.
Which ship fuels are you looking into in your research?
“Recently, I’ve been concentrating on what are known as electrofuels, i.e. fuels that are made from hydrogen produced through the electrolysis of water and combined with carbon dioxide or nitrogen. If we add carbon dioxide to hydrogen, we can produce e.g. methane, methanol or diesel, and if we add nitrogen we can produce ammonia. Previously, we looked at LNG, methanol and biofuels where we studied production costs and conducted life cycle analyses. Next we’ll start taking a closer look at ammonia, pure hydrogen and electric drive.”
What are the advantages of methanol as a fuel?
“One advantage is that methanol is liquid at room temperature and normal pressures, unlike e.g. hydrogen and LNG. Methanol is a small molecule, and it takes relatively little energy to produce compared to diesel, for example. Also, methanol has a lower risk of climate impact compared to e.g. methane, which is a powerful greenhouse gas in itself. The combustion of methanol produces mainly carbon dioxide and water vapour. NOx and particulates are also formed, in about the same quantities as the combustion of LNG. How much is actually formed depends largely on the engine concept used.”
And what are the disadvantages?
“Methanol contains less energy per unit mass than e.g. diesel, and this requires larger tanks. It is of course a toxic substance even when in contact with the skin, and must be handled appropriately, so special safety procedures are necessary when using methanol as a fuel. And it shares a problem with all electrofuels – it’s expensive to produce. Electricity is necessary both for the production of hydrogen gas and the actual methanol, and the equipment itself requires major investment, at least for the time being. It also calls for large quantities of carbon dioxide. Renewable sources of carbon dioxide are of course preferable from a climate standpoint, but their availability is limited. Another alternative is to capture carbon dioxide from the atmosphere, but that technology is expensive and not yet fully developed.”



